Lentivirus titer and structure
The problem
Being able to quickly and comprehensively monitor the quality of your lentiviral prep from crude sample all the way through your purification process is key for rapid process development. Current methods are limited in their ability to handle crude viral sample, making it impossible to get an accurate lentiviral titer, monitor viral stability, and measure non-viral contaminants in early stage samples.
The most common method for measuring physical lentivirus titer is a p24 ELISA assay. Since p24 protein is relatively abundant in free solution, this assay is seriously limited because it can't tell the difference between p24 used in lentivirus capsids and soluble p24 not associated with a virus. This results in inaccurate lentiviral titers when the concentration of p24 protein doesn't scale along with the quantity of the virus - especially a problem in crude samples with high concentrations of soluble p24.
When it comes to detecting contaminants, ELISAs also can't give any info on lentivirus aggregation and the high specificity of ELISAs leave you in the dark about any empty viral particles that don't contain p24. All this means the p24 ELISA falls short of delivering comprehensive answers on the composition and integrity of your viral prep, and the effectiveness of your lentivirus production and purification process.
The right tool for the job
Leprechaun allows lentiviruses to be measured at all stages during production and links up data on protein structure with a physical lentivirus titer - all with no purification needed. Simultaneously measure viral titer, virus size, and tell the difference between the virus you want and all the other particles that can be missing VSVG or p24. Leprechaun also measures contaminating particles like extracellcular vesiscles (EVs), and differentiates viral p24 from soluble p24. All that info adds up to knowing exactly what’s in your sample, which is essential if you’re planning to use your lentivirus for CAR-T production.
The proof
Leprechaun quantifies 5 key components of any lentivirus prep on a single particle basis to show if each individual particle has a lentivirus capsid, has the right surface proteins, is an aggregate, or is just an EV. First, particles are immuno-captured by three different antibodies onto the surface of one Luni consumable.
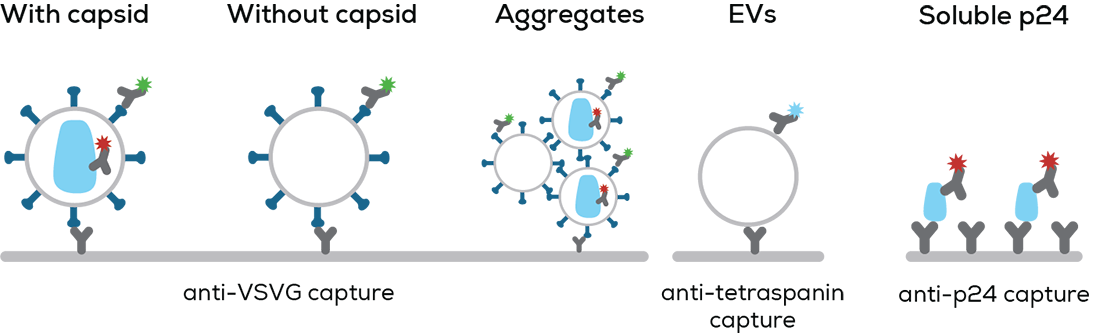
Then Leprechaun uses interferometry to size up captured particles one-by-one, between 35 and 200 nm diameter. Finally, immunofluorescence microscopy shows off exactly which particles are the lentiviruses you’re after – the ones that are the right size and have VSVG and p24 present. Anything else that doesn't have the right combo of size and detected protein is a damaged, incorrectly assembled lentivirus, an EV, or contaminants like soluble p24 and EVs.
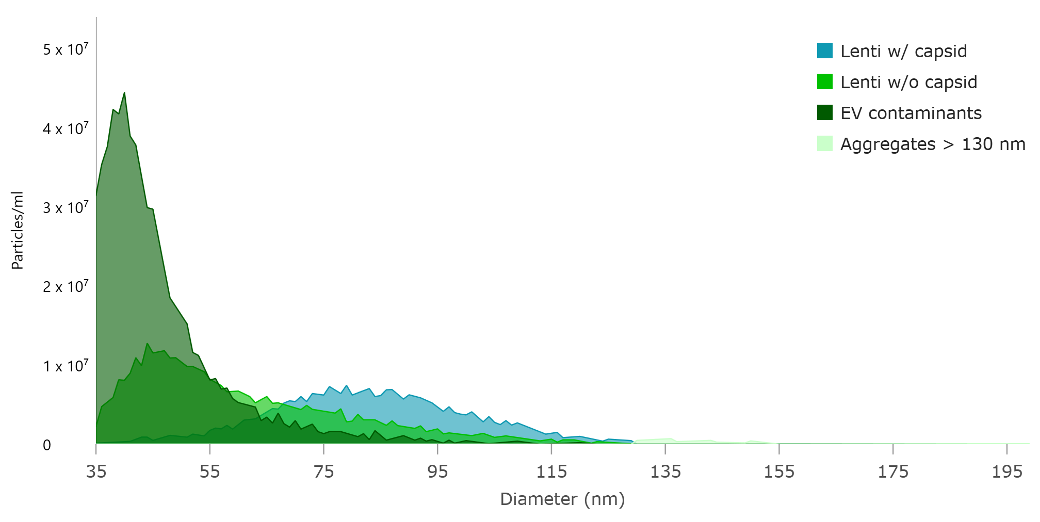
Any time you check on your lentivirus with Leprechaun, from crude samples at early stages of production to your purified virus ready to use for CAR-T manufacture, contaminants will stick out as the wrong size or by having the wrong structural components. Tracking lentivirus titer and all kinds of contaminants throughout your process paints the full picture of lentivirus stability and gives you the readout you need to optimize your lentivirus production and purification.

Leprechaun
Leprechaun is the only system that hunts down viral titer by double-checking if particles are the right size and have the right structure. Count up your lentiviruses with capsids in crude or pure samples. Make your own luck and follow Leprechaun straight to the viral titer you’ve been looking for without the noise from trickster particles that can throw you off the trail.
Want more info?
Want to learn more about how Leprechaun gives you lentivirus titer and structure info?